

Interestingly, though, this simple orbital view doesn't suffice for carbon.Ĭarbon has four valence electrons: two in its 2 s orbital and two in its 2 p orbital (one in each of two directional 2 p orbitals, to be precise-2 p x and 2 p y, for instance).
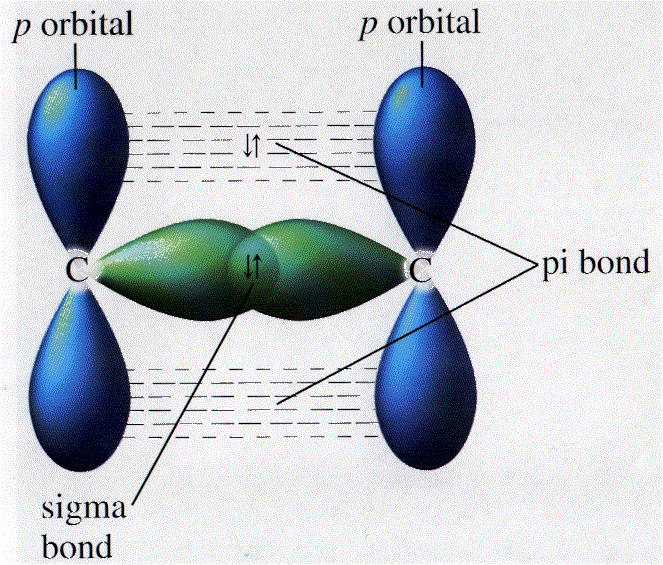
This type of bond, where the orbitals overlap along the line formed by the two nuclei is called a sigma (?) bond. The distance between the nuclei (the bond length) in this case is 74 picometers, or 0.000000000074 meters. At this point, the energy of the two-hydrogen "system" is minimized, and the hydrogen molecule is stable. The hydrogen nuclei approach until the constructive interference of the electron wave functions is balanced by the repulsion of the positively charged nuclei (as well as the repulsion of the electrons, to some extent).
#SIGMA BONDS FULL#
This picture is simple enough: each hydrogen has a half-filled 1 s orbital, and the overlapping orbitals yield a single full 1 s orbital (i.e., a full valence shell) shared by both atoms. In the case of two hydrogen atoms, the spherical 1 s orbitals overlap to form an ellipsoid-like orbital, as shown below (drawing not to scale), that represents the orbital of the bonded molecule H 2. This constructive interference occurs between the nuclei of the atoms as they approach one another, and it is related to the fact that the negatively charged electrons are attracted to the area between the positively charged nuclei owing to the electrostatic force. If electrons act like waves in orbitals, then, presumably, those orbitals can overlap in such a manner that the waves constructively interfere (as opposed to destructive interference). Nevertheless, a qualitative application of quantum theory can help explain how bonding occurs. So what is happening between two carbon atoms or a carbon atom and a hydrogen atom to cause them to "stick" together?īecause we cannot see atoms, we cannot be entirely sure. Note that electrons seem to behave more like waves than like particles.


 0 kommentar(er)
0 kommentar(er)
